Pure Suspension Cell Culture Medium Market to Accelerate Across APAC, Europe, USA & Saudi Arabia Through 2035
Global demand for scalable bioprocessing drives rapid growth in pure suspension cell culture media across biopharma and cell therapy sectors.
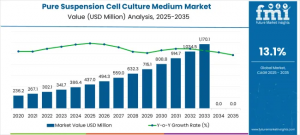
FRANCE, November 12, 2025 /EINPresswire.com/ -- The Pure Suspension Cell Culture Medium Market is positioned for substantial expansion, forecasted to rise from USD 437.0 million in 2025 to USD 1.49 billion by 2035, reflecting a CAGR of 13.1%. The growth is driven by intensified biopharmaceutical production, expanding cell therapy pipelines, and increasing adoption of high-density suspension culture systems in research and commercial manufacturing. These systems enable scalable production of vaccines, monoclonal antibodies, and gene therapies while maintaining cell integrity and yielding higher process efficiencies.
Explore trends before investing – request a sample report today!
https://www.futuremarketinsights.com/reports/sample/rep-gb-26665
Market Expansion Across Key Regions
North America remains a leading contributor, underpinned by advanced biologics manufacturing and well-defined regulatory pathways. The United States continues to drive high-value adoption through rapid commercialization of cell and gene therapies. Europe demonstrates balanced growth, supported by strong infrastructure in Germany, the United Kingdom, and France, where investments in regenerative medicine and GMP-certified research continue to rise.
Meanwhile, Asia Pacific shows the strongest acceleration, particularly in China, India, and South Korea, where governments are prioritizing domestic biopharmaceutical capacity building. Saudi Arabia and broader GCC regions are increasingly investing in biotechnology innovation zones, signaling emerging demand for standardized, scalable cell culture solutions to support local therapeutic manufacturing.
Key Market Drivers Influencing Demand
A primary driver of market growth is the intensifying global pipeline of monoclonal antibody and protein-based therapeutics. Biopharmaceutical manufacturers are prioritizing media formulations that enable consistent, high-viability cell growth in suspension systems, reducing production time and improving yield reliability. The market also benefits from advanced manufacturing strategies leveraging perfusion bioreactors, automated monitoring systems, and quality-by-design platforms to ensure reproducibility and regulatory compliance at commercial scale.
Moreover, rising investment in personalized medicine and regenerative therapies is creating demand for highly optimized media types designed around specific cell lineage performance characteristics. This shift is reshaping procurement strategies across R&D divisions, contract development and manufacturing organizations (CDMOs), and large-scale therapeutic producers.
Segmental Performance and Product Adoption
The serum-containing suspension medium segment is projected to account for 61.4% of market share in 2025 due to its long-standing validation and compatibility across multiple bioprocessing platforms. However, serum-free and chemically defined media are expected to show faster future adoption as manufacturers aim to reduce variability and meet regulatory requirements for traceability.
Biopharmaceutical applications dominate demand at 68.3% share, supported by commercialization of cell-based interventions and increasing R&D investment in advanced immunotherapies. Research institutions and university laboratories also contribute to demand growth, leveraging suspension culture technologies for oncology, virology, and vaccine development studies.
Outlook Through 2035
The strongest growth performance is anticipated between 2033 and 2035, where accelerated adoption of cell-based therapies drives higher consumption of high-quality media formulations. APAC markets are expected to represent significant incremental market share due to expanding biomanufacturing footprints and state-supported investments in life sciences infrastructure.
Buy Report Now – Click Here to Purchase the Report:
https://www.futuremarketinsights.com/checkout/26665
Latest Healthcare IT Reports
Organ Preservation Solution Market
https://www.futuremarketinsights.com/reports/organ-preservation-solution-market
Digital Pathology Displays Market
https://www.futuremarketinsights.com/reports/digital-pathology-displays-market
Remote ICU Monitoring System Market
https://www.futuremarketinsights.com/reports/remote-icu-monitoring-system-market
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates:- https://www.futuremarketinsights.com/reports/brochure/rep-gb-26665
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.